How Could You Genetically Modify An Animal So That It Would Distinguish More Shades Of Green
Cytogenetics entered the molecular era with the introduction of in situ hybridization, a process that allows researchers to locate the positions of specific DNA sequences on chromosomes. Since the start in situ hybridization experiments in 1969 (Gall & Pardue, 1969), many variations of the procedure have been adult, and its sensitivity has increased enormously. Today, most in situ hybridization procedures apply fluorescent probes to find Deoxyribonucleic acid sequences, and the process is unremarkably referred to as FISH (fluorescence in situ hybridization). A diverseness of FISH procedures are available to cytogeneticists, who use them to diagnose many types of chromosomal abnormalities in patients. The success of FISH, and all other methods of in situ hybridization, depends on the remarkable stability of the DNA double helix.
In Situ Hybridization Is Used to Localize DNA Sequences on Chromosomes
In 1953, James Watson and Francis Crick described the all-encompassing network of hydrogen bonds that hold together the ii antiparallel strands in the DNA double helix (Watson & Crick, 1953). Today, even schoolchildren know that adenine on ane Deoxyribonucleic acid strand binds to thymine on the complementary DNA strand, and that cytosine as well binds to guanine. Because of the many hydrogen bonds formed between these bases, the double helix is a remarkably stable structure. Moreover, if the hydrogen bonds that hold the helix together are broken with heat or chemicals, the helix is able to re-grade when conditions get more favorable. This ability of the DNA helix to re-course, or renature, provides the basis for molecular hybridization.
In molecular hybridization, a labeled Deoxyribonucleic acid or RNA sequence is used as a probe to place or quantify the naturally occurring counterpart of the sequence in a biological sample. In the 1960s, researchers Joseph Gall and Mary Lou Pardue realized that molecular hybridization could be used to identify the position of DNA sequences in situ (i.e., in their natural positions inside a chromosome). In fact, in 1969, the ii scientists published a landmark paper demonstrating that radioactive copies of a ribosomal DNA sequence could exist used to detect complementary Deoxyribonucleic acid sequences in the nucleus of a frog egg. Since those original observations, many refinements have increased the versatility and sensitivity of the procedure to the extent that in situ hybridization is now considered an essential tool in cytogenetics.
Fluorescent Probes Are Introduced

© 2005 Nature Publishing Grouping Speicher, M. R. et al. The new cytogenetics: blurring the boundaries with molecular biology. Nature Reviews Genetics 6, 784 (2005). All rights reserved. ![]()
Shortly after Gall and Pardue's piece of work, fluorescent labels chop-chop replaced radioactive labels in hybridization probes because of their greater safety, stability, and ease of detection (Rudkin & Stollar, 1977). In fact, near electric current in situ hybridization is washed using FISH procedures (Trask, 2002; Speicher & Carter, 2005). Detecting a DNA sequence can be compared to looking for a needle in a haystack, with the needle being the DNA sequence of interest and the haystack being a set up of chromosomes. This search is made much easier if the investigator has a powerful "magnet"—in this instance, a fluorescent copy of the Dna sequence of involvement. Hybridization occurs when the "magnet" meets the "needle"; this requires both a probe and a target, as shown in Effigy 1. In the effigy, the probe sequence, often a piece of cloned DNA, is shown in reddish. The target DNA—chromosomes on a drinking glass slide—is shown in blue (in the correct column). Hydrogen bonds that join the two strands of the DNA helix are represented by black lines.
The starting time stride in the process is to brand either a fluorescent copy of the probe sequence (Figure 1b, middle cavalcade) or a modified copy of the probe sequence that tin can be rendered fluorescent later in the process (Effigy 1b, left column). Adjacent, before any hybridization tin occur, both the target and the probe sequences must be denatured with rut or chemicals (Figure 1c). This denaturation step is necessary in order for new hydrogen bonds to form between the target and the probe during the subsequent hybridization footstep. The probe and target sequences are then mixed together (Effigy 1d), and the probe specifically hybridizes to its complementary sequence on the chromosome. If the probe is already fluorescent (middle column), information technology volition exist possible to find the site of hybridization directly. In other cases (left column), an additional step may exist needed to visualize the hybridized probe. Hybrids formed between the probes and their chromosomal targets can be detected using a fluorescent microscope.
When investigators design a FISH experiment, they need to consider whether the sensitivity and resolution needed for the experiment lie inside the technical limits of fluorescence microscopy. Sensitivity depends on the light-gathering ability of the particular microscope, which determines whether small-scale target sequences, which are more hard to see than big target sequences, can be detected. Resolution refers to the ability to distinguish betwixt two points along the length of a chromosome. Ultimately, light microscopy cannot resolve objects that are separated by less than 200–250 nm, the lower limit of the visible light spectrum. With these technical limits in mind, investigators too need to consider the conformation of Dna within the chromosome. Metaphase chromosomes are thousands of times more compacted than interphase chromosomes, which in turn are at to the lowest degree ten times more compacted than naked Deoxyribonucleic acid. (Remember that 1 iii.four nm turn of the DNA helix corresponds to 10 base pairs of DNA.) When all these factors are considered together, investigators typically expect to obtain resolution in the range of megabases for positions on metaphase chromosomes and resolution in the range of tens of thousands of kilobases for interphase chromosomes.
Using FISH to Identify the Positions of Genes
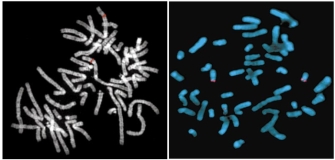
© 2001 Nature Publishing Group Cheung, V. M. et al. Integration of cytogenetic landmarks into the draft sequence of the man genome. Nature 409, 954 (2001). All rights reserved. ![]()
FISH provides a powerful tool for identifying the location of a cloned Dna sequence on metaphase chromosomes. Figure 2a shows the results of a typical FISH experiment, in which a cloned DNA sequence was hybridized to normal metaphase chromosomes. Crimson bands are detected at hybridization sites on two homologous chromosomes, which can be identified past their characteristic banding patterns. Closer examination shows that each red band actually consists of two spots, corresponding to the two sister chromatids in a mitotic chromosome. A skilled cytogeneticist would be able to use these hybridization data together with the banding pattern to place the probe sequence inside a few megabases of other known genes on the chromosome.
Historically, FISH and other in situ hybridization results played a chief role in mapping genes on human being chromosomes. Results from these experiments were collected and compiled in databases, and this information proved useful during the annotation phase of the Human Genome Project (HGP). At present that the HGP is complete, investigators rarely use in situ hybridization but to identify the chromosomal location of a human gene. (In species for which the genome has not been sequenced, nonetheless, FISH and related in situ hybridization methods continue to provide important information for mapping the positions of genes on chromosomes.) Currently, homo FISH applications are principally directed toward clinical diagnoses.
Diagnosing Chromosomal Abnormalities Using Karyotypes and FISH
FISH and other in situ hybridization procedures are important in the clinical diagnosis of various chromosomal abnormalities, including deletions, duplications, and translocations. Figure 2b shows ane example in which investigators used FISH together with standard karyotyping to clarify a patient translocation. The hybridization probe corresponded to a segment of chromosome xix that was suspected to include the translocation breakpoint. Iii areas of hybridization are apparent in the fluorescent paradigm. I spot corresponds to the patient'due south normal copy of chromosome nineteen (nl19), and the other 2 spots correspond to the altered, or derived (der), versions of chromosomes xi and 19 that were produced during the translocation. Thus, investigators were able to use the information both to narrow down the breakpoint region on chromosome nineteen and to identify the second chromosome involved in the translocation.
The hybridization probe used in Figure 2b was ane of thousands of bacterial artificial chromosome (BAC) clones from the HGP that take been fabricated available to the scientific community. Today, cytogeneticists are able to use all-encompassing HGP clone resources to precisely identify the sites of chromosomal rearrangements that appear in karyotypes. In fact, a consortium of scientists has mapped over vii,000 Deoxyribonucleic acid clones from the HGP to specific bands on human being chromosomes (BAC Research Consortium, 2001). At least one clone is available for every megabase segment of chromosomal Dna. (The just exception is the Y chromosome, because it is relatively gene-poor.)
Using Collections of FISH Probes to "Paint" Unabridged Chromosomes

© 2014 Panels a) and b) modified from © 2000 Cambridge University Press. McNeil, N. & Ried, T. Novel molecular cytogenetic techniques for identifying complex chromosomal rearrangements: technology and applications in molecular medicine. Good Reviews in Molecular Medicine (online: September 2000). All rights reserved. ![]()
The detection of chromosome rearrangements with site-specific probes (Figure 2b) can exist a lengthy endeavour, especially if circuitous rearrangements have occurred or if the rearranged regions are difficult to identify by their banding patterns in a karyotype. Fortunately, cytogeneticists now take the option of using multifluor FISH, or spectral karyotyping, to quickly scan a prepare of metaphase chromosomes for potential rearrangements (Speicher et al., 1996; Schrock et al., 1996). Multifluor FISH generates a karyotype in which each chromosome appears to be painted with a different color. Each "paint" is really a drove of hybridization probes for sequences that span the length of a particular chromosome.
With multifluor FISH, investigators start prepare a drove of DNA sequences to exist used as probes for each chromosome. In Figure 3a, the probe chromosomes take been physically separated from ane another by menstruum cytometry. (Today, investigators would probably use commercially bachelor DNA collections for each chromosome.) In the side by side step, the Dna samples are labeled with combinations of fluorochromes that produce a unique color for each chromosome. (The Cot-one DNA step in the figure removes repetitive Dna sequences [e.g., centromeric Deoxyribonucleic acid] that would demark to all chromosomes.) The fluorescent hybridization probes are then combined with and hybridized to metaphase chromosomes. Figure 3b shows images of interphase and metaphase chromosomes as they would appear through a microscope after hybridization. To human eyes, several of the metaphase chromosomes appear to accept the same color, but digital processing of the image would distinguish spectral differences between the chromosomes. A normal human chromosome (Effigy 3b) will have a uniform color forth its length, but a rearranged chromosome will have a striped appearance.
Although chromosome paints let rapid assessment of large chromosomal changes in metaphase spreads, the resolution of the method is express. Thus, while chromosome painting allows investigators to quickly identify chromosomes involved in translocations and to identify large deletions and/or duplications, pocket-sized deletions and duplications volition not exist detectable. If investigators need more detailed information about the bodily sequences involved in chromosomal rearrangements, they need to follow upwardly with site-specific probes, as previously described (Figure 2).
Using FISH to Analyze Interphase Chromosomes

© 1986 Cold Spring Harbor Laboratory Press Modified with permission from Gray, J. W., et al. Menstruum karyotyping and sorting of homo chromosomes. Cold Jump Harbor Symposia on Quantitative Biological science 51, 141–149 (1986). All rights reserved. Plot courtesy of Ger van den Engh, Found of Systems Biological science. All rights reserved. ![]()
Since the introduction of FISH, cytogeneticists have been able to clarify interphase chromosomes besides equally the metaphase chromosomes used in karyotypes (Trask, 2002). This offers a real practical advantage, in that cells do not need to be cultured for several days or weeks before chromosomes can be prepared for assay. In addition, FISH tin be used to analyze chromosomes from specimens such as solid tumors, which are of great clinical interest simply do non divide frequently. Some other useful characteristic of FISH is that researchers are able to simultaneously monitor multiple sites if the hybridization probes have been labeled with different fluorophores.
Figure four shows ii examples of how interphase FISH can be used to diagnose chromosome abnormalities. Figure 4a shows an interphase nucleus from a patient with Charcot-Marie-Tooth illness (CMT) type 1A (Lupski et al., 1991). CMT type 1A is a relatively common neurological status caused by a duplication in a gene on chromosome 17 that encodes one of the proteins in the myelin sheath that surrounds nervus axons. In Figure 4a, the patient's cell has been hybridized with a ruby-red-labeled probe corresponding to a sequence within the duplicated region, forth with a green probe corresponding to a sequence on chromosome 17 that lies outside of the duplicated region. From the 2 green signals, it is possible to locate two copies of chromosome 17 within the nucleus. One chromosome has the normal configuration, while the second, der(17), contains the duplicated region, which is evident from two nearby ruby signals. The figure also serves to illustrate another important feature of interphase FISH. Considering interphase chromatin is about 10,000 times less compacted than mitotic chromatin, it is possible to resolve the duplicated regions on der(17) every bit discrete points. This small duplication would have been difficult to resolve in mitotic chromosomes.
Figure 4b shows a FISH analysis that was used to notice the presence of a chromosomal translocation in a patient suffering from chronic myelogenous leukemia (Tkachuk et al., 1990). In most cases of this disease, a segment of chromosome 9 that contains the ABL proto-oncogene fuses with the breakpoint cluster region (BCR) on chromosome 22 during a reciprocal translocation. The derived chromosome 22, or der(22), also known as the Philadelphia chromosome, contains a BCR-ABL fusion gene in which the powerful BCR promoter drives synthesis of the ABL oncogene transcript, leading to cancer. Figure 4b demonstrates that BCR-ABL fusions tin be readily identified past FISH when a green-labeled hybridization probe flanking BCR is practical together with a ruby-red-labeled probe flanking ABL. In this epitome, the normal copies of chromosomes 9 and 22 are detected as cerise and light-green spots, respectively. On the other mitt, the Philadelphia chromosome is visible as a complex fused spot, which appears to take a fundamental yellowish region with red and dark-green subregions on either side. (In fluorescence microscopy, yellowish is indicative of very close proximity of scarlet and green probes, such that they announced to overlap.) The intricate substructure of the fused spot is detectable in interphase chromosomes, but information technology would not exist resolved in a similar FISH assay of metaphase chromosomes. Thus, two-colour interphase FISH provides a sensitive method for analyzing chromosome fusion events without the need for a prior jail cell culture.
Another research application of interphase FISH makes use of chromosome-specific paints to obtain information about the arrangement of chromosomes within the nucleus. Figure 2a (upper left) shows an interphase nucleus that has been stained with chromosome-specific paints. One tin see from the figure that the chromosomes occupy distinct territories within the nucleus. By creatively combining chromosome-specific probes with gene-specific probes and antibodies, investigators tin can apply FISH to provide exciting new insights near nuclear architecture.
Additional Applications of FISH in the Clinic and Research Laboratory
Exciting new applications of FISH that extend its range go on to exist developed. For example, cytogeneticists now employ comparative genomic hybridization to detect quantitative differences, like re-create number variations, in the chromosomes of their patients. Recently, investigators accept also been able to increase the resolution of FISH past using stretched chromatin fibers (Parra & Windle, 1993) or microarrays equally the target. With tools such as these, cytogenetics has been able to move from studying whole chromosomes on the macroscopic scale, to studying the Dna of which these chromosomes consist.
References and Recommended Reading
BAC Resource Consortium. Integration of cytogenetic landmarks into the draft sequence of the human genome. Nature 409, 953–958 (2001) (link to commodity) Gall, J. K., & Pardue, M. L. Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proceedings of the National Academy of Sciences 63, 378 – 383 (1969) Lupski, J. R., et al. Deoxyribonucleic acid duplication associated with Charcot-Marie-Tooth disease blazon 1A. Cell 66, 219 – 232 (1991) doi:ten.1016/0092-8674(91)90613-iv Parra, I., & Windle, B. High resolution visual mapping of stretched Dna by fluorescent hybridization. Nature Genetics v, 17 – 21 (1993) doi:ten.1038/ng0993-17 (link to article) Rudkin, Thousand. T., & Stollar, B. D. High resolution of Deoxyribonucleic acid-RNA hybrids in situ by indirect immunofluorescence. Nature 265, 472 – 474 (1977) doi:10.1038/265472a0 (link to commodity) Schrock, Due east., et al. Multicolor spectral karyotyping of human chromosomes. Science 273, 494 – 497 (1996) doi:x.1126/science.273.5274.494 Speicher, G. R., Ballard, S. G., & Ward, D. C. Karyotyping human being chromosomes by combinatorial multi-fluor FISH. Nature Genetics 12, 368 – 375 (1996) doi:10.1038/ng0496-368 (link to article) Speicher, M. R., & Carter, Northward. P. The new cytogenetics: Blurring the boundaries with molecular biological science. Nature Reviews Genetics 6, 782 – 792 (2005) doi:x.1038/nrg1692 (link to article) Tkachuk, D. C., et al. Detection of BCR-ABL fusion in chronic myelogenous leukemia past in situ hybridization. Science 250, 559 – 562 (1990) doi:10.1126/scientific discipline.2237408 Trask, B. J. Human cytogenetics: 46 chromosomes, 46 years and counting. Nature Reviews Genetics iii, 769 – 778 (2002) doi:10.1038/nrg905 (link to article) Watson, J. D., & Crick, F. H. C. Molecular construction of nucleic acids: A structure for deoxyribose nucleic acid. Nature 171, 737 – 738 (1953) doi:x.1038/171737a0 (link to commodity)
Source: http://www.nature.com/scitable/topicpage/fluorescence-in-situ-hybridization-fish-327
Posted by: hinesthessfy63.blogspot.com

0 Response to "How Could You Genetically Modify An Animal So That It Would Distinguish More Shades Of Green"
Post a Comment